REPAIR YOUR LEAKING
HEART VALVE.
RECLAIM YOUR LIFE.
Think heart-related problems are
just something you have to live with?
Think again.
DISCUSS ALL TREATMENT OPTIONS WITH YOUR DOCTOR
Your doctor can describe the risks and benefits and help you decide which option is right for you.
Patients often experience a significant
improvement in quality of life
I wake up and I feel good, I feel like doing more today
- Scott, MitraClip™ patient*
*This testimonial relates an account of an individual’s response to the treatment. This patient’s account is genuine, typical and documented. However, it does not provide any indication, guide, warranty or guarantee as to the response other persons may have to the treatment. Responses to the treatment discussed can and do vary and are specific to the individual patient.
Why have 150,000+ people chosen MitraClip™?
Saves Lives
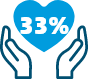
Relative risk reduction in
mortality1
FEWER HOSPITALIZATIONS

Relative risk reduction in
heart failure hospitalization1
FEEL BETTER

More likely to experience a
large improvement in
quality of life2
About Mitral Regurgitation
What is a leaking heart valve (mitral regurgitation)?
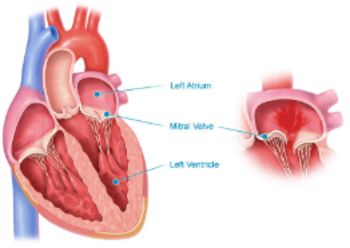
First, it’s important to understand that the mitral valve is located between your heart’s two left chambers, the atrium and ventricle. The mitral valve has two flaps of tissue, called leaflets, that open and close to ensure that blood flows in only one direction.
When the mitral valve fails to close completely, blood leaks backward inside your heart—that’s a condition called mitral regurgitation, commonly called leaky valve or heart murmur.
One type of mitral regurgitation is called primary (or degenerative) mitral regurgitation. It can be related to age, a birth defect, or underlying heart disease.
The other type is called secondary (or functional) mitral regurgitation. It is caused by heart disease that leads to an enlarged left ventricle.
What can happen if my leaking heart valve isn’t treated?
The mitral valve not working properly places an extra burden on your heart and lungs. Over time, some people may develop an enlarged heart because it has to work harder to pump blood through the body. If it is not treated, this condition can cause other, more serious problems to your heart, such as heart failure, a condition that occurs when your heart can’t pump enough blood to meet the needs of your body. Learn more about that now.
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional medical advice. Consult with a physician or qualified healthcare provider for appropriate medical advices.
A leaky heart valve reduces your quality of life.
In some cases, people with a leaky valve (mitral regurgitation) may never experience symptoms of heart
failure. But over time, heart failure can worsen when the heart is unable to pump enough blood to meet the
body’s demands. Its signs are important to recognize and discuss with your doctor.

Shortness of breath

Fatigue

Dry, hacking cough

Excessive urination

Fainting

Swollen feet or ankles

Inability to exercise

Decrease in appetite
A leaky heart valve reduces your quality of life.
In some cases, people with a leaky valve (mitral regurgitation) may never experience symptoms of heart failure. But over time, heart failure can worsen when the heart is unable to pump enough blood to meet the body’s demands. Its signs are important to recognize and discuss with your doctor.
- Shortness of breath
- Fatigue
- Dry, hacking cough
- Excessive urination
- Fainting
- Swollen feet or ankles
- Inability to exercise
- Decrease in appetite
What is MitraClip™?
MitraClip™ is a simple procedure to fix your mitral valve.
During the procedure, doctors access the mitral valve with a thin tube (called a catheter) that is guided through a vein in your leg to reach your heart.
A small implanted clip is attached to your mitral valve to help it close more completely. This helps to restore normal blood flow through your heart.

Your doctor may refer to it as transcatheter mitral valve repair (TMVr) or as minimally invasive, which is very different from traditional surgery. Unlike surgery, the MitraClipTM procedure does not require opening the chest and temporarily stopping the heart.
As you can see, it’s smaller than a dime, which allows it to be placed through a catheter-based procedure.
Most patients experience improvement in their leaky valve-related symptoms and quality of life soon after the procedure.
It’s a proven therapy.
On average, this minimally invasive procedure takes only 1-3 hours. Plus, most patients go home within 1-3 days!5
After this quick procedure, people have had less risk of mortality, had fewer hospitalizations, and felt much better than with just medication.5

of proven safety

patients have been treated worldwide

FAQ
- What is a leaky valve/mitral regurgitation?
Leaky valve (technically called mitral regurgitation) is a common condition that affects one of the valves in your heart most commonly—the mitral valve.
About 1 in 10 people aged 75 and older have moderate or severe mitral regurgitation.1
To determine if you have mitral regurgitation and to assess the function and condition of your heart and mitral valve, your cardiologist may perform diagnostic evaluations, including:- Taking a chest x-ray and/or ultrasound to see the size and shape of your heart and evaluate your lungs
- Evaluating you for symptoms of congestive heart failure (such as shortness of breath or fatigue) or other related heart conditions
Find a MitraClip™ center near you, or get a brochure.
- How will I know if MitraClip™ is right for me?
You will be evaluated by a specially trained heart team, including an interventional cardiologist and a clinical cardiologist, who will review your medical history and perform a variety of tests. There are several factors they will take into consideration when deciding whether or not you are eligible for open-heart surgery and therefore a possible candidate for MitraClip™ therapy, such as your age, frailty, and the condition of your heart.
Your doctor may decide that the MitraClip™ procedure is not appropriate for you if you:- Cannot tolerate medications that thin the blood or prevent blood clots from forming
- Have an active infection or inflammation of the mitral valve
- Have mitral valve disease as a result of rheumatic fever
- Have a blood clot in your heart or in the vessels that carry blood from the lower body to the heart
Your doctor will discuss with you whether any of these issues will prevent you from having the MitraClip™ procedure. An evaluation of your heart will also confirm whether your heart valve anatomy allows for successful placement of the device.
Find a MitraClip™ center near you, or get a brochure.
- What is MitraClip™ made of?
The MitraClip™ device is a small metal clip covered with a polyester fabric that is implanted on your mitral valve. The clip is inserted through a catheter, without the need to temporarily stop your heart. There can be up to 4 different clip sizes used to tailor your clip size to your valve.
Find a MitraClip™ center near you, or get a brochure.
- What are the benefits of the MitraClip™ procedure?
More than 150,000 patients have been treated with the MitraClip™ device worldwide.* More than 30,000 of these patients have been followed through multiple studies and registries.†
The results are impressive:
SAVES LIVES: 33% reduction in relative risk of mortality.1
FEWER HOSPITALIZATIONS: 51% relative risk reduction in heart failure hospitalization.1
HELPS PATIENTS FEEL BETTER: 2.5x more likely to experience a large improvement in quality of life.2
And it’s proven to be safe:
MINIMALLY INVASIVE: This procedure does not require opening the chest or temporarily stopping the heart.
QUICK PROCEDURE: The implantation procedure typically lasts 1 to 3 hours.4
SHORT HOSPITAL STAY: Patients are usually released from the hospital within 1 to 3 days, significantly less time compared to surgery.4
Patients who were studied 5 years after the MitraClip™ procedure continued to experience improvement in their heart function, quality of life, and ability to perform day-to-day tasks.Find a MitraClip™ center near you, or get a brochure.
- How do I prepare for the MitraClip™ procedure?
In the days before your procedure, it is important that you:
- Take all of your prescribed medications
- Tell your doctor if you are taking any other medications
- Make sure your doctor knows of any allergies you have
- Follow all instructions given to you by your doctor or nurse
Learn more about how the MitraClip™ procedure works.
Find a MitraClip™ center near you, or get a brochure.
- How soon after having the MitraClip™ procedure will I start to feel the effects?
Clinical data from patients who underwent the MitraClip™ procedure demonstrate an immediate reduction of mitral regurgitation. You should experience a significant improvement in your leaky valve–related symptoms and quality of life soon after your procedure. It is important to discuss what to expect following the procedure with your transcatheter mitral valve repair (TMVr) heart team.
Find a MitraClip™ center near you, or get a brochure.
- Will I be able to tell that the MitraClip™ device is in my heart?
No, you will not be able to feel the implant.
Find a MitraClip™ center near you, or get a brochure.
- Can I have an MRI if I have a MitraClip™ device?
It is possible that you may be able to have aa magnetic resonance imaging (MRI) scan performed. However, you must inform your MRI facility and technician that you have a MitraClip™ device. After the procedure you will be given an implant card that describes what types of MRIs you will be able to receive. If the test can be performed, they may need to make certain adjustments to ensure your safety.
Find a MitraClip™ center near you, or get a brochure.
- Will I see improvements with MitraClip™ over medication alone?
In a clinical study called COAPT, heart failure patients with mitral regurgitation who were treated with MitraClip™ and medication experienced improvements over those treated with medication alone.
The COAPT trial demonstrated that MitraClip™:- Saves lives: 33% relative risk reduction in mortality
- Reduces hospitalizations for heart failure: 51% relative risk reduction in heart failure hospitalization
- Can help you feel better: 2.5x more likely to experience a large improvement in quality of life
- Is safe and well tolerated: 96.6% freedom from device-related complications at 12 months
Find a MitraClip™ center near you, or get a brochure.
- How do I find a MitraClip™ center near me?
Find a MitraClip™ center near you, or get a brochure.
Stay updated about MitraClip™
And don’t forget to download a brochure.
IMPORTANT SAFETY INFORMATION
What is MitraClip™ Therapy approved for?
Available by prescription only.
MitraClip therapy is a minimally invasive procedure approved for treating patients with clinically significant mitral regurgitation due to either (a) a deteriorated mitral valve but the patient is at very high risk for surgery, or (b) an improperly functioning mitral valve resulting from heart failure, with poor pumping of the blood by an enlarged heart, and who have reached maximal tolerance to medications. Patients should work with their doctor and a multidisciplinary heart team, which should include a heart surgeon and cardiologist with experience treating heart failure, to confirm their surgical risk or optimal medications. The heart team will determine if the patient meets the indications for the MitraClip procedure.
Who should not have the MitraClip Procedure?
Patients that have any of the following conditions should not have the MitraClip Procedure: inability to tolerate or are allergic or hypersensitive to anti-coagulants, anti-platelet therapies, nickel, titanium, cobalt, chromium, polyester, or contrast dye; have inflammation or rheumatic disease of the valve; or have blood clots inside the heart or blood vessels (inferior vena cava, femoral vein).
What can happen to me during the MitraClip Procedure?
As with most medical procedures, MitraClip placement has risks, including inappropriate device placement, device movement from its implanted site, and failed or difficult delivery or retrieval of the device once implanted. Your physician will determine if you fall within the labeled indication for the MitraClip Procedure.
Who is more at risk during the MitraClip Procedure?
Even though MitraClip Therapy is a minimally invasive medical procedure, it carries risks, and some patients may be at a higher risk than others. If you have either a weak heart that may need support during the procedure or a rotated heart from prior heart surgery, talk to your doctor to weigh the additional risks to the benefits of the MitraClip procedure as the safety and effectiveness has not been tested in these patients.
What are the possible complications associated with the MitraClip procedure?
The MitraClip Procedure carries risks which include, but are not limited to: Allergic reactions to the implant materials or medications used during or after the procedure; Tissue damage at the puncture (entry) site such as wound reopening, reaction to the catheter, bleeding, air bubbles, tissue or nerve injury; Inflammation, buildup of fluid or blood in the sac surrounding the heart, and complications that may require more interventions or heart surgery; Mitral valve complications including device dislodgement, entanglement with chords, narrowing of the mitral valve, continuing backflow of blood through the mitral valve during heart contraction, and inflammation; Abnormal heart rhythm, stroke (resulting from blood clot or burst vessel in the brain) or transient stroke, high or low blood pressure; Multiple organ failure, death, pain; Complications related to the echocardiographic imaging such as irritation or perforation of the throat.
Talk to your doctor to learn more about the risks associated with MitraClip Therapy and visit the full Important Safety Information if you'd like to review the full list of complications.
The information provided is not intended for medical diagnosis or treatment or as a substitute for professional medical advice. Consult with a physician or qualified healthcare provider for appropriate medical advices.
Photo(s) on file at Abbott.
Unless otherwise specified, all product names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates.
No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
MAT-2010280 v4.0 | Item approved for U.S. use only
The data referenced below and within this website are specific to Functional (Secondary) Mitral Regurgitation:
1Mack M, et al. COAPT: Three-year outcomes from a randomized trial of transcatheter mitral valve leaflet approximation in patients with heart failure and secondary mitral regurgitation. Presented at TCT 2019.
2Arnold SV et al. Health status after transcatheter mitral valve repair in heart failure and secondary mitral regurgitation. JACC Mar 2019, 25951; DOI: 10.1016/j.jacc.2019.02.010.
3Cioffi G, et al. Eur J Heart Fail. 2005;7(7):1112-1117
4Mayo Clinic. Mitral valve regurgitation. Accessed August 17, 2021.
5Lim DS et al. Contemporary Outcomes with MitraClip (NTRXTR) System In Primary Mitral Regurgitation Results From The Global EXPAND, ACC 2020.
