TAILORED.
OPTIMIZED.
PROVEN.
MITRACLIP™ G4.
Watch to learn more about the features and benefits of Mitraclip G4

EXPANDED PORTFOLIO OF CLIP SIZES*
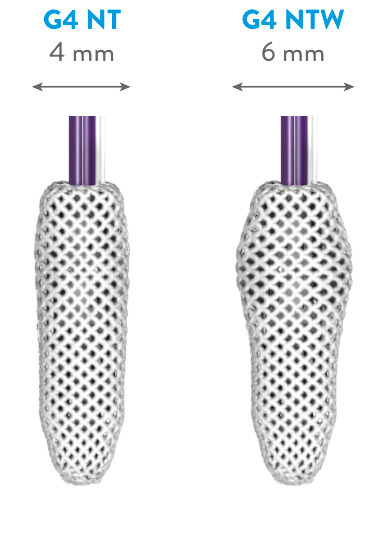

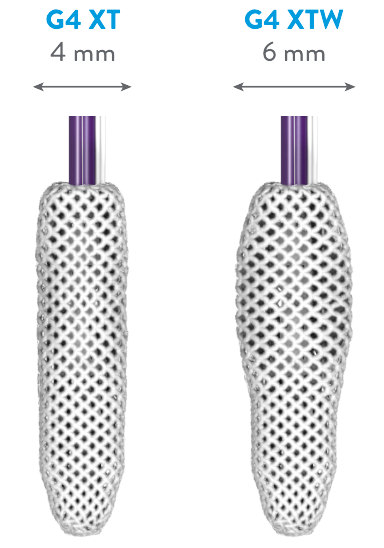
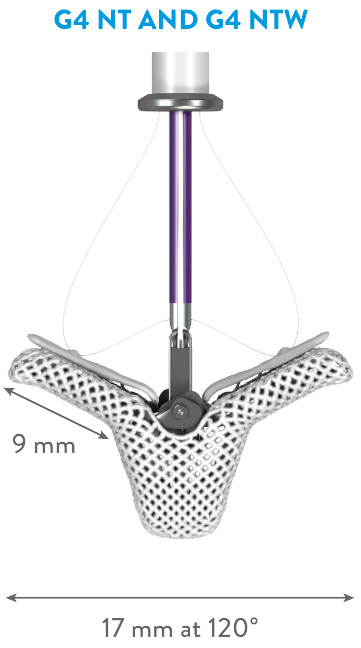
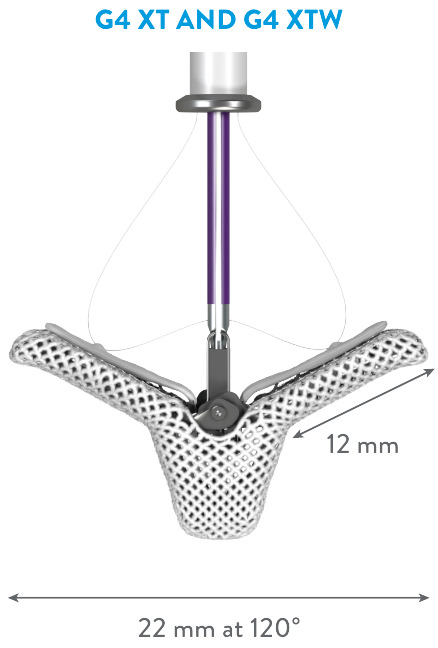
*MitraClip G4 IFU.
HIGHEST MITRAL REGURGITATION (MR) REDUCTION TO DATE1
DESIGNED TO TAILOR AND FURTHER REDUCE MR WITH A SINGLE CLIP†
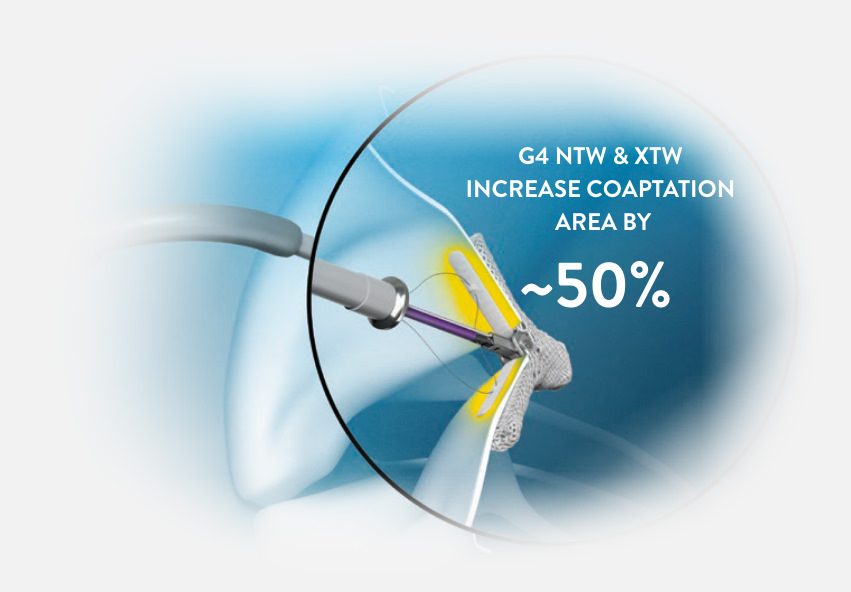
“ALLOWS US TO TREAT PATIENTS WITH 1 CLIP MORE OFTEN THAN BEFORE.”
—Echocardiographer with 6 years of MitraClip experience, commenting on MitraClip G4‡
†Tests performed by and data on file at Abbott.
TREAT MORE PATIENTS WITH MORE OPTIONS1,2
SIGNIFICANT MR REDUCTION IN SIMPLE AND COMPLEX ANATOMIES2
“Long-arm clip use was
associated with improved MR
reduction for severe
baseline MR, smaller
annular dimensions, larger
prolapse gaps, and complex
disease in primary MR.”
—Cardiac Surgeon with over 10 years of MitraClip experience‡
MitraClip successfully treats a broad range of valve anatomies in real world1,3
Nearly 1 in 5 patients have valve anatomies considered complex3
Valve anatomies included: presence of severely degenerative leaflets, wide flail gaps or widths, calcified landing zone, wide jet, primary jet outside of A2/P2, and more.3
Abbreviations: PMR, primary mitral regurgitation; SMR, secondary mitral regurgitation.
Controlled gripper actuation (CGA) to confirm and optimize leaflet grasping
CGA gives the option to move the grippers simultaneously or independently
Test performed by and data on file at Abbott.
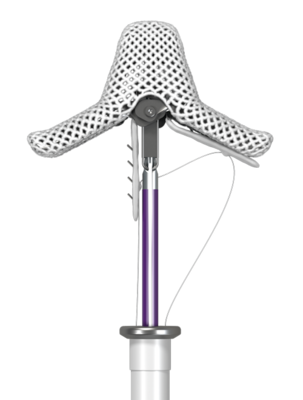
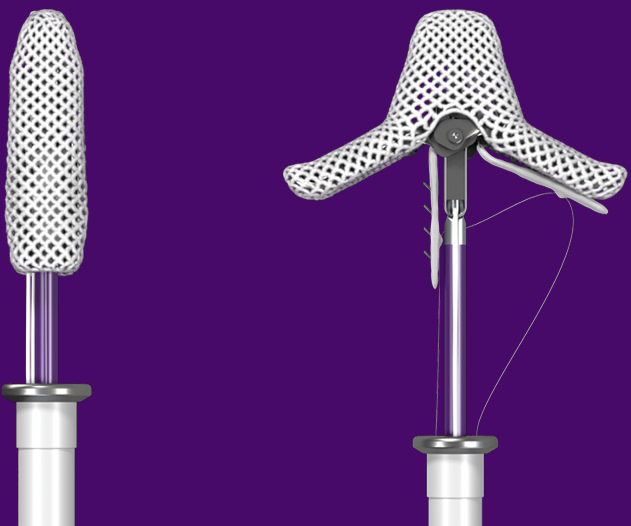
Advanced delivery system for precise steering
MitraClip G4 features an advanced delivery system specifically for mitral valve repair that delivers precise and controlled steering.‡
- Straight trajectory into the left ventricle
- Stable clip arm orientation when crossing
- Enables expanded target area of the transseptal puncture
- Facilitates left atrial pressure monitoring
Simplified TMVr* procedure
MitraClip G4 is designed for increased procedural efficiency.
Reduction in implanted clip rate per procedure
- 1 implanted clip in 61% of patients‡
- 7% reduction in clip implanted per procedure‡
Shortened procedural time
- 39-minute average device time‡
- 15% shorter procedural time‡
Simplified procedural steps
- 40% reduction in system preparation steps*
- Simplified deployment with reduced number of steps*
- Synchronous clip and gripper line detachment in a single step
*MitraClip G4 IFU
**TMVr is now also referred to as TEER (transcatheter edge-to-edge repair)
‡Tests performed by and data on file at Abbott.
The MitraClip System

MitraClip G4 Clip Delivery System (CDS) and Steerable Guide Catheter (SGC)
LEARN ABOUT THE LATEST MITRACLIP CLINICAL DATA

MAT-2410455 v2.0 | Item approved for U.S. use only
‡ The testimonial does not provide any indication, guide, warranty, or guarantee as to the response patients may have to the treatment or effectiveness of the product or therapy in discussion. Opinions about the treatment discussed can and do vary and are specific to the individual’s experience and might not be representative of others.
- Rottbauer WD. Contemporary Clinical Outcomes with MitraClip™ (NTR/XTR) System: Core-lab Echo Results from +1000 Patient the Global EXPAND Study. Data presented at PCR 2020.
- Maisano F. Clip Selection Strategy and Outcomes with MitraClip™ (NTR/XTR): Evidence-Based Recommendations from the Global EXPAND Study. Data presented at PCR 2020.
- Maisano F, Torracca L, Oppizzi M, et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998;13(3):240-246.
- Alfieri O, Maisano F. An effective technique to correct anterior mitral leaflet prolapse. J Card Surg. 1999;14(6):468-470.
- Maisano F, Schreuder JJ, Oppizzi M, et al. The double-orifice technique as a standardized approach to treat mitral regurgitation due to severe myxomatous disease: surgical technique. Eur J Cardiothorac Surg. 2000;17(3):201-205.
- Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122(4):674-681.
REQUEST AN ABBOTT REP
Rx Only
Important Safety Information
MITRACLIP™ CLIP DELIVERY SYSTEM
- The MitraClip™ G4 System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation.
- The MitraClip™ G4 System, when used with maximally tolerated guideline-directed medical therapy (GDMT), is indicated for the treatment of symptomatic, moderate-to-severe or severe secondary (or functional) mitral regurgitation (MR; MR ≥ Grade III per American Society of Echocardiography criteria) in patients with a left ventricular ejection fraction (LVEF) ≥ 20% and ≤ 50%, and a left ventricular end systolic dimension (LVESD) ≤ 70 mm whose symptoms and MR severity persist despite maximally tolerated GDMT as determined by a multidisciplinary heart team experienced in the evaluation and treatment of heart failure and mitral valve disease.
Contraindications
The MitraClip G4 System is contraindicated in patients with the following conditions: Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or post procedural anti-platelet regime; Patients with known hypersensitivity to clip components (nickel / titanium, cobalt, chromium, polyester), or with contrast sensitivity; Active endocarditis of the mitral valve; Rheumatic mitral valve disease; Evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus.
Potential Complications and Adverse Events
The following ANTICIPATED EVENTS have been identified as possible complications of the MitraClip G4 procedure: Allergic reactions or hypersensitivity to latex, contrast agent, anaesthesia, device materials (nickel / titanium, cobalt, chromium, polyester), and drug reactions to anticoagulation, or antiplatelet drugs, Vascular access complications which may require transfusion or vessel repair including: wound dehiscence, catheter site reactions, Bleeding (including ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage), Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, vascular occlusion, Emboli (air thrombotic material, implant, device component); Peripheral Nerve Injury; Lymphatic complications; Pericardial complications which may require additional intervention, including: Pericardial effuse on, Cardiac tamponade, Pericarditis; Cardiac complications which may require additional interventions or emergency cardiac surgery, including: Cardiac perforation, Atrial septal defect; Mitral valve complications, which may complicate or prevent later surgical repair, including: Chordal entanglement / rupture, Single Leaflet Device Attachment (SLDA), Thrombosis, Dislodgement of previously implanted devices, Tissue damage, Mitral valve stenosis, Persistent or residual mitral regurgitation, Endocarditis; Cardiac arrhythmias (including conduction disorders, atrial arrhythmias, ventricular arrhythmias); Cardiac ischemic conditions (including myocardial infarction, myocardial ischemia, and unstable / stable angina); Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post procedure pulmonary embolism); Stroke / Cerebrovascular accident (CVA) and Transient Ischemic Attack (TIA); System organ failure: Cardio-respiratory arrest, Worsening heart failure, Pulmonary congestion, Respiratory dysfunction / failure / atelectasis, Renal insufficiency or failure, Shock (including cardiogenic and anaphylactic); Blood cell disorders (including coagulopathy, hemolysis, and Heparin Induced Thrombocytopenia (HIT)); Hypotension / hypertension; Infection including: Urinary Tract Infection (UTI), Pneumonia, Septicemia; Nausea / vomiting; Chest pain; Dyspnea; Edema; Fever or hyperthermia; Pain; Death; Fluoroscopy, Transesophageal echocardiogram (TEE) and Transthoracic echocardiogram (TTE) -related complications: Skin injury or tissue changes due to exposure to ionizing radiation, Esophageal irritation; Esophageal perforation, Gastrointestinal bleeding.
