MITRACLIP™ TRANSCATHETER MITRAL VALVE REPAIR (TMVr)* IS FIRST AND FOREMOST IN MITRAL REGURGITATION (MR) TREATMENT
Watch the MitraClip TMVr procedure video animation
*TMVr is now also referred to as TEER (transcatheter edge-to-edge repair)
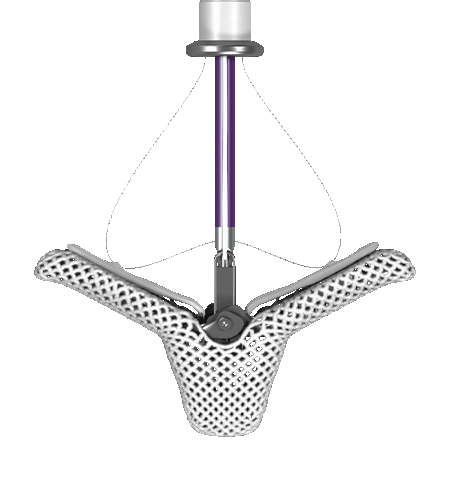
A MINIMALLY INVASIVE PROCEDURE: MITRACLIP THERAPY
For your Mitral Regurgitation patients who are ineligible for surgery or select heart failure patients who remain symptomatic despite guideline-directed medical therapy (GDMT), MitraClip is an important treatment option that can offer improved quality of life.
A MINIMALLY INVASIVE PROCEDURE: MITRACLIP
For your Mitral Regurgitation patients who are ineligible for surgery or select heart failure patients who remain symptomatic despite guideline-directed medical therapy (GDMT), MitraClip is an important treatment option that can offer improved quality of life. It’s the only mitral valve device shown to improve survival in patients with secondary MR.
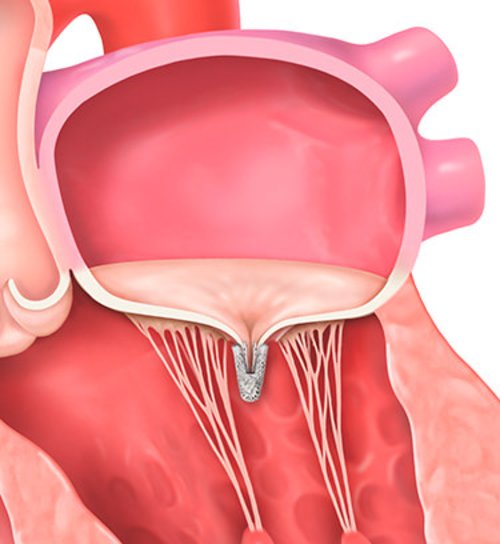
MitraClip placement
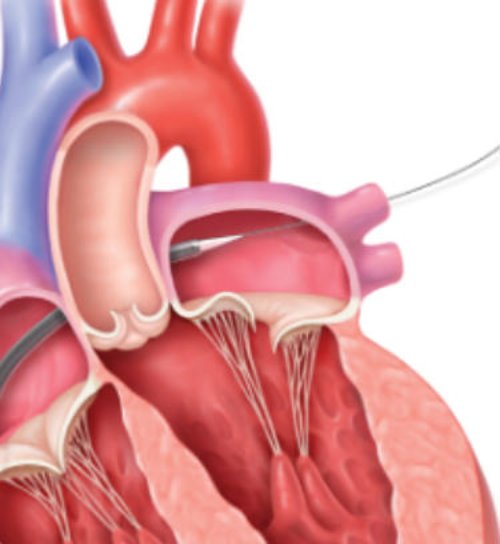
Transseptal crossing, guide, and delivery system insertion
Steering and advancing clip into left atrium to line of coaptation
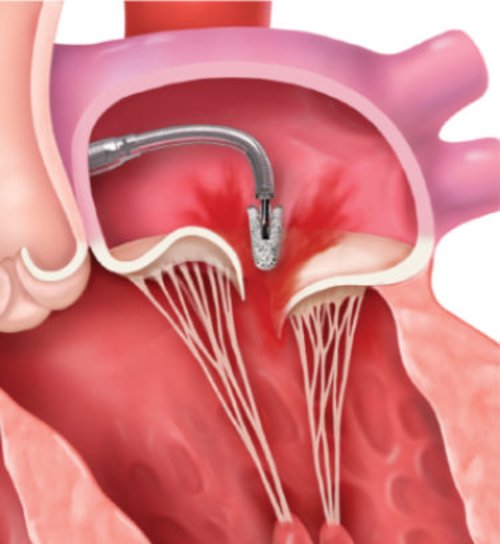
Leaflet grasping, assessment, and hemodynamic measurements
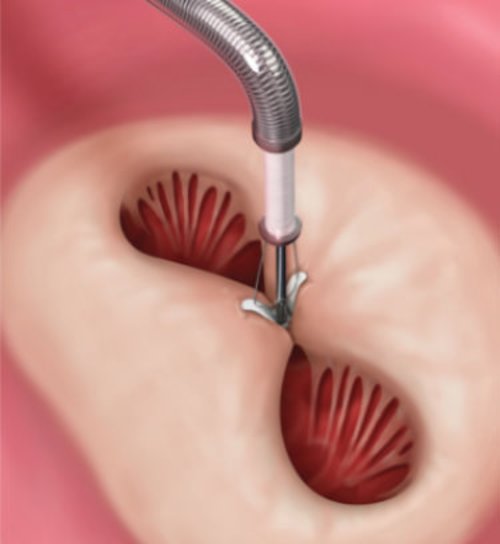
Deployment and removal after successful placement
MitraClip has treated 100,000+ patients with a leaky heart valve, like Bill
“It’s been years since I’ve felt like this.”
—Bill, MitraClip patient*
*This testimonial relates an account of an individual’s response to treatment. This patient’s account is genuine, typical, and documented. However, it does not provide any indication, guide, warranty, or guarantee as to the response other persons may have to the treatment. Responses to the treatment discussed can and do vary and are specific to the individual patient.
MAT-2410455 v2.0 | Item approved for U.S. use only
- Sorajja P, Vemulapalli S, Feldman T, et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. 2017;70(19):2315-2327.
- Kar S. Core-Lab Adjudicated Contemporary Clinical Outcomes at 1 Year with MitraClip™ (NTR/XTR) System from Global EXPAND Study. Data presented at TCT 2020.
- Mack M. COAPT: 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients with Heart Failure. JACC Mar 2021.
REQUEST AN ABBOTT REP
Rx Only
Important Safety Information
MITRACLIP™ CLIP DELIVERY SYSTEM
- The MitraClip™ G4 System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation.
- The MitraClip™ G4 System, when used with maximally tolerated guideline-directed medical therapy (GDMT), is indicated for the treatment of symptomatic, moderate-to-severe or severe secondary (or functional) mitral regurgitation (MR; MR ≥ Grade III per American Society of Echocardiography criteria) in patients with a left ventricular ejection fraction (LVEF) ≥ 20% and ≤ 50%, and a left ventricular end systolic dimension (LVESD) ≤ 70 mm whose symptoms and MR severity persist despite maximally tolerated GDMT as determined by a multidisciplinary heart team experienced in the evaluation and treatment of heart failure and mitral valve disease.
Contraindications
The MitraClip G4 System is contraindicated in patients with the following conditions: Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or post procedural anti-platelet regime; Patients with known hypersensitivity to clip components (nickel / titanium, cobalt, chromium, polyester), or with contrast sensitivity; Active endocarditis of the mitral valve; Rheumatic mitral valve disease; Evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus.
Potential Complications and Adverse Events
The following ANTICIPATED EVENTS have been identified as possible complications of the MitraClip G4 procedure: Allergic reactions or hypersensitivity to latex, contrast agent, anaesthesia, device materials (nickel / titanium, cobalt, chromium, polyester), and drug reactions to anticoagulation, or antiplatelet drugs, Vascular access complications which may require transfusion or vessel repair including: wound dehiscence, catheter site reactions, Bleeding (including ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage), Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, vascular occlusion, Emboli (air thrombotic material, implant, device component); Peripheral Nerve Injury; Lymphatic complications; Pericardial complications which may require additional intervention, including: Pericardial effuse on, Cardiac tamponade, Pericarditis; Cardiac complications which may require additional interventions or emergency cardiac surgery, including: Cardiac perforation, Atrial septal defect; Mitral valve complications, which may complicate or prevent later surgical repair, including: Chordal entanglement / rupture, Single Leaflet Device Attachment (SLDA), Thrombosis, Dislodgement of previously implanted devices, Tissue damage, Mitral valve stenosis, Persistent or residual mitral regurgitation, Endocarditis; Cardiac arrhythmias (including conduction disorders, atrial arrhythmias, ventricular arrhythmias); Cardiac ischemic conditions (including myocardial infarction, myocardial ischemia, and unstable / stable angina); Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post procedure pulmonary embolism); Stroke / Cerebrovascular accident (CVA) and Transient Ischemic Attack (TIA); System organ failure: Cardio-respiratory arrest, Worsening heart failure, Pulmonary congestion, Respiratory dysfunction / failure / atelectasis, Renal insufficiency or failure, Shock (including cardiogenic and anaphylactic); Blood cell disorders (including coagulopathy, hemolysis, and Heparin Induced Thrombocytopenia (HIT)); Hypotension / hypertension; Infection including: Urinary Tract Infection (UTI), Pneumonia, Septicemia; Nausea / vomiting; Chest pain; Dyspnea; Edema; Fever or hyperthermia; Pain; Death; Fluoroscopy, Transesophageal echocardiogram (TEE) and Transthoracic echocardiogram (TTE) -related complications: Skin injury or tissue changes due to exposure to ionizing radiation, Esophageal irritation; Esophageal perforation, Gastrointestinal bleeding.
