MitraClip™ 5-year data
in primary mitral
regurgitation (PMR)
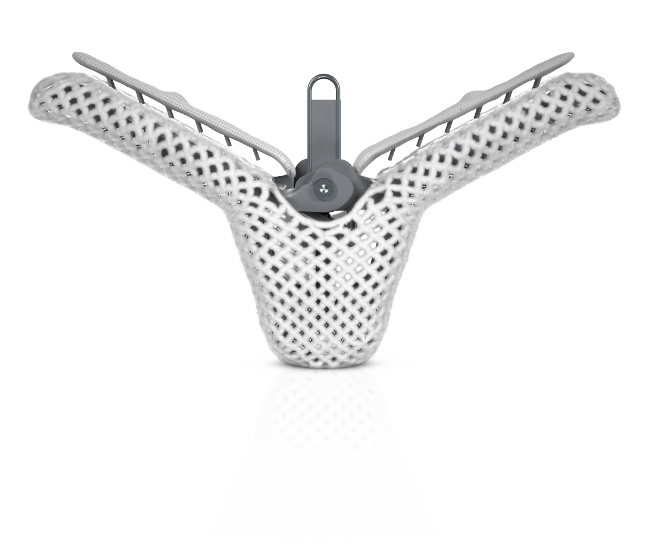
The only transcatheter mitral valve repair (TMVr)* device with proven durable outcomes and 17+ years of clinical experience**
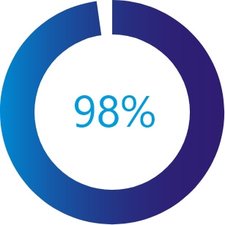
98% of patients were successfully implanted with the MitraClip device1
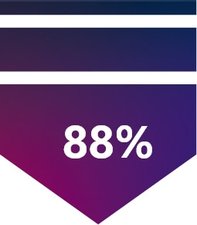
88% had severity reduction to mitral regurgitation (MR) ≤ 2+ at 5 years4

91% had New York Heart Association (NYHA) Functional Class I or II at 5 years2

MitraClip has shown an impact on left ventricular remodeling3
*TMVr is now also referred to as TEER (transcatheter edge-to-edge repair)
**Data on file at Abbott.
MAT-2410455 v2.0 | Item approved for U.S. use only
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379(24):2307-2318.
- Feldman T, Kar S, Elmariah S, et al; EVEREST II Investigators. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66(25):2844-2854.
- Sorajja P, Vemulapalli S, Feldman T, et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. 2017;70(19):2315-2327.
- Feldman T. The EVEREST II REALISM Continued Access Study: Five-year outcomes in high surgical risk patients. Data presented at PCR 2018.
REQUEST AN ABBOTT REP
Rx Only
Important Safety Information
MITRACLIP™ CLIP DELIVERY SYSTEM
- The MitraClip™ G4 System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation.
- The MitraClip™ G4 System, when used with maximally tolerated guideline-directed medical therapy (GDMT), is indicated for the treatment of symptomatic, moderate-to-severe or severe secondary (or functional) mitral regurgitation (MR; MR ≥ Grade III per American Society of Echocardiography criteria) in patients with a left ventricular ejection fraction (LVEF) ≥ 20% and ≤ 50%, and a left ventricular end systolic dimension (LVESD) ≤ 70 mm whose symptoms and MR severity persist despite maximally tolerated GDMT as determined by a multidisciplinary heart team experienced in the evaluation and treatment of heart failure and mitral valve disease.
Contraindications
The MitraClip G4 System is contraindicated in patients with the following conditions: Patients who cannot tolerate, including allergy or hypersensitivity to, procedural anticoagulation or post procedural anti-platelet regime; Patients with known hypersensitivity to clip components (nickel / titanium, cobalt, chromium, polyester), or with contrast sensitivity; Active endocarditis of the mitral valve; Rheumatic mitral valve disease; Evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus.
Potential Complications and Adverse Events
The following ANTICIPATED EVENTS have been identified as possible complications of the MitraClip G4 procedure: Allergic reactions or hypersensitivity to latex, contrast agent, anaesthesia, device materials (nickel / titanium, cobalt, chromium, polyester), and drug reactions to anticoagulation, or antiplatelet drugs, Vascular access complications which may require transfusion or vessel repair including: wound dehiscence, catheter site reactions, Bleeding (including ecchymosis, oozing, hematoma, hemorrhage, retroperitoneal hemorrhage), Arteriovenous fistula, pseudoaneurysm, aneurysm, dissection, perforation / rupture, vascular occlusion, Emboli (air thrombotic material, implant, device component); Peripheral Nerve Injury; Lymphatic complications; Pericardial complications which may require additional intervention, including: Pericardial effuse on, Cardiac tamponade, Pericarditis; Cardiac complications which may require additional interventions or emergency cardiac surgery, including: Cardiac perforation, Atrial septal defect; Mitral valve complications, which may complicate or prevent later surgical repair, including: Chordal entanglement / rupture, Single Leaflet Device Attachment (SLDA), Thrombosis, Dislodgement of previously implanted devices, Tissue damage, Mitral valve stenosis, Persistent or residual mitral regurgitation, Endocarditis; Cardiac arrhythmias (including conduction disorders, atrial arrhythmias, ventricular arrhythmias); Cardiac ischemic conditions (including myocardial infarction, myocardial ischemia, and unstable / stable angina); Venous thromboembolism (including deep vein thrombosis, pulmonary embolism, post procedure pulmonary embolism); Stroke / Cerebrovascular accident (CVA) and Transient Ischemic Attack (TIA); System organ failure: Cardio-respiratory arrest, Worsening heart failure, Pulmonary congestion, Respiratory dysfunction / failure / atelectasis, Renal insufficiency or failure, Shock (including cardiogenic and anaphylactic); Blood cell disorders (including coagulopathy, hemolysis, and Heparin Induced Thrombocytopenia (HIT)); Hypotension / hypertension; Infection including: Urinary Tract Infection (UTI), Pneumonia, Septicemia; Nausea / vomiting; Chest pain; Dyspnea; Edema; Fever or hyperthermia; Pain; Death; Fluoroscopy, Transesophageal echocardiogram (TEE) and Transthoracic echocardiogram (TTE) -related complications: Skin injury or tissue changes due to exposure to ionizing radiation, Esophageal irritation; Esophageal perforation, Gastrointestinal bleeding.
